Pertuzumab
Overview
Pertuzumab is a humanized monoclonal antibody designed to target the HER2 receptor, which plays a critical role in the development of certain aggressive breast cancers. It works by binding to a different site on the HER2 receptor than trastuzumab, thereby preventing receptor dimerization and downstream signaling that drive tumor growth. Pertuzumab has become a vital therapy in the management of HER2-positive breast cancer, both in early-stage and metastatic settings. It is administered in combination with trastuzumab and chemotherapy to provide synergistic inhibition of HER2 signaling, improving clinical outcomes. This therapy is given intravenously under the supervision of oncology specialists, and its use has been associated with significant improvements in progression-free survival and overall survival in breast cancer patients.
Background and Date of Approval
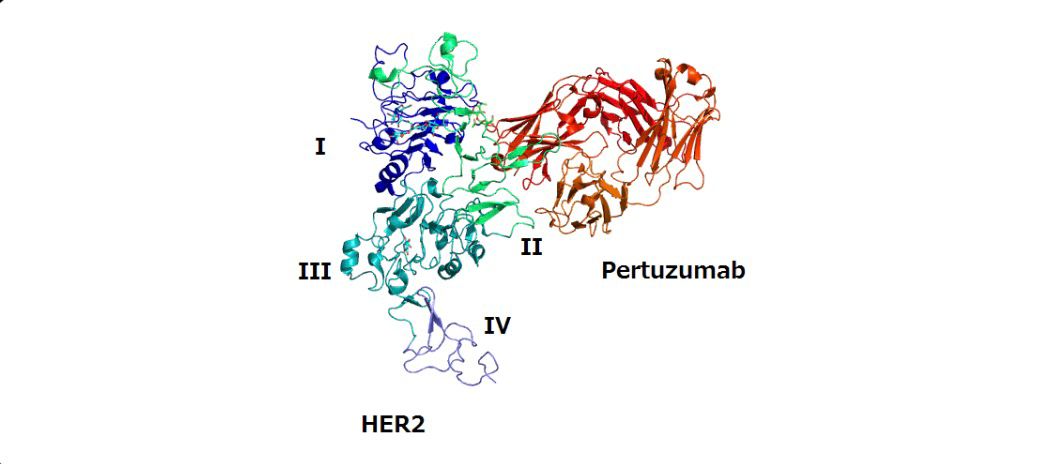
The development of pertuzumab emerged from the need to provide more effective blockade of HER2-driven signaling pathways. Unlike trastuzumab, which binds to domain IV of HER2, pertuzumab targets domain II, preventing HER2 from pairing with other HER receptors such as HER3, which is one of the strongest signaling dimers. Clinical studies demonstrated that this dual blockade with trastuzumab and pertuzumab provided superior efficacy compared to trastuzumab alone. The pivotal CLEOPATRA trial, published in 2012, showed remarkable improvements in progression-free survival and overall survival in patients with HER2-positive metastatic breast cancer treated with the pertuzumab regimen. Based on these findings, the US FDA approved pertuzumab in 2012 for use in combination with trastuzumab and docetaxel in HER2-positive metastatic breast cancer. Subsequently, it gained approval in early breast cancer settings, including neoadjuvant and adjuvant therapy. The European Medicines Agency and regulatory authorities worldwide have also approved pertuzumab, recognizing its role as a key component of HER2-directed treatment.
Uses
Pertuzumab is indicated for the treatment of HER2-positive metastatic breast cancer in combination with trastuzumab and chemotherapy in patients who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease. It is also approved for neoadjuvant use in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer at high risk of recurrence. In addition, pertuzumab is recommended in the adjuvant setting to reduce the risk of recurrence in patients with high-risk HER2-positive breast cancer. The combination of pertuzumab with trastuzumab forms a dual HER2 blockade approach that has become a standard of care, significantly improving survival outcomes in eligible patients.
Administration
Pertuzumab is administered as an intravenous infusion. The recommended loading dose is 840 mg, followed by maintenance doses of 420 mg given every three weeks. It is typically given in combination with trastuzumab and a taxane, most commonly docetaxel, as part of the treatment regimen. The infusion is administered under medical supervision with careful monitoring for infusion-related reactions. Dosing adjustments may be required based on patient tolerance, cardiac function monitoring, and treatment-related adverse effects. Therapy should only be initiated and supervised by healthcare professionals experienced in oncology and chemotherapy administration.
Side Effects
The most frequently reported side effects of pertuzumab include diarrhea, alopecia, rash, nausea, fatigue, and neutropenia. Patients may also experience fever, decreased appetite, and mucosal inflammation. Infusion-related reactions such as chills and fever can occur during or shortly after the first infusion, though these are generally manageable with supportive care. Most side effects are mild to moderate, but ongoing monitoring and supportive treatment are essential to ensure tolerability and adherence to therapy.
Warnings
Pertuzumab carries a risk of serious adverse effects, the most notable being cardiotoxicity, particularly when used in combination with trastuzumab. Patients require baseline and regular monitoring of left ventricular ejection fraction during treatment. Other serious risks include hypersensitivity reactions, severe diarrhea leading to dehydration, and risk of neutropenic fever when used with chemotherapy. Pertuzumab is contraindicated in patients with known hypersensitivity to the drug or its excipients. Use during pregnancy is associated with fetal harm, and effective contraception is required during treatment and for several months after the last dose.
Precautions
Precautions include regular monitoring of cardiac function due to the increased risk of left ventricular dysfunction. Patients should also be monitored for severe gastrointestinal toxicity and hematologic abnormalities when used in combination with chemotherapy. Dose delays or discontinuation may be required in the event of severe adverse events. Pertuzumab has limited known drug interactions, but caution should be exercised when combining it with other cardiotoxic agents or overlapping toxicities from chemotherapy. Special care is required for pregnant women, nursing mothers, and patients with pre-existing cardiac conditions.
Expert Tips
Healthcare professionals prescribing pertuzumab should ensure comprehensive cardiac monitoring before and throughout treatment. Patients should be counseled about the risk of diarrhea, rash, and infusion-related reactions, and provided with supportive care measures to manage these adverse effects. Pharmacists should ensure that dosing schedules are correctly followed and that pertuzumab is not interchanged with trastuzumab, as both drugs have distinct dosing and infusion requirements. Coordinated care with oncology nurses and cardiology specialists can improve patient safety and optimize treatment outcomes.
