Polatuzumab Vedotin
Overview
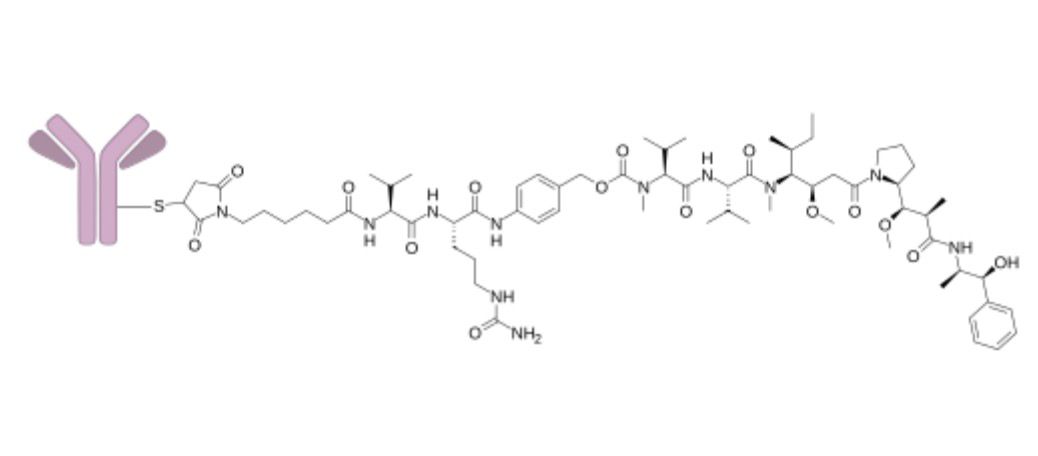
Polatuzumab Vedotin is an antibody-drug conjugate (ADC) directed against CD79b, used in the treatment of diffuse large B-cell lymphoma (DLBCL). The antibody component binds to CD79b on malignant B-cells and delivers a cytotoxic payload, monomethyl auristatin E (MMAE), into the cell to interrupt microtubule formation, causing cell death. Polatuzumab Vedotin offers a way to deliver chemotherapy directly to cancer cells, which helps improve treatment specificity and reduce systemic toxicity compared to non-targeted cytotoxics. It is administered by intravenous infusion in combination with other agents under supervised settings.
Background and Date of Approval
Polatuzumab Vedotin was granted accelerated approval by the U.S. Food and Drug Administration in 2019 for adult patients with relapsed or refractory DLBCL after at least two prior therapies, when used in combination with bendamustine and rituximab. This approval was based on the clinical trial GO29365 which showed improved response rates and survival in the treated group compared with control. In 2023 the FDA approved Polatuzumab Vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP) for previously untreated DLBCL and high-grade B-cell lymphoma in patients with an elevated prognostic risk score, based on the POLARIX trial. Regulatory approvals in Europe (EMA) similarly recognize its use in relapsed/refractory DLBCL and provide dosing guidelines for both first-line and salvage settings.
Uses
Polatuzumab Vedotin indications include treatment of adult patients with diffuse large B-cell lymphoma that has relapsed or is refractory to at least two prior lines of therapy, in combination with bendamustine and rituximab. It is also indicated as first-line therapy in combination with R-CHP (rituximab, cyclophosphamide, doxorubicin, prednisone) for patients with previously untreated DLBCL or high-grade B-cell lymphoma who have an intermediate to high risk score. Treatment is approved only for patients who are suitable for these combination regimens and under supervision in oncology settings.
Administration
Polatuzumab Vedotin is administered intravenously at a standard dose of 1.8 mg per kilogram of body weight every 21 days. When used in combination with bendamustine and rituximab, dosing is for six cycles. In the first-line R-CHP regimen, dosing is similarly scheduled every three weeks for six cycles followed by maintenance or rituximab monotherapy as per protocols. Infusions must be given under supervision, and premedication may be required to reduce infusion reactions. Dose adjustments are recommended in patients experiencing severe adverse effects, particularly hematologic toxicities or neuropathy.
Side Effects
Common side effects of Polatuzumab Vedotin when used in approved regimens include neutropenia, thrombocytopenia, anemia, peripheral neuropathy, fatigue, diarrhea, decreased appetite, fever, and pneumonia. These side effects are often manageable with standard supportive care measures, dose reductions, or treatment interruptions when needed. Onset of neuropathy may be gradual and monitored throughout therapy.
Warnings
Serious risks associated with Polatuzumab Vedotin include severe infections, infusion-related reactions, tumor lysis syndrome, and significant myelosuppression leading to neutropenia and thrombocytopenia. There is potential for severe peripheral neuropathy which can be disabling. Polatuzumab Vedotin is contraindicated in patients with known hypersensitivity to the drug or its components. Women should avoid breastfeeding during treatment and for a period after. Liver function should be monitored, and use in patients with hepatic impairment requires caution.
Precautions
Prior to administration, baseline blood counts, liver function, and risk of infection should be assessed. Monitoring of blood counts is essential with each cycle given the risk of myelosuppression. Concurrent use of strong CYP3A4 inhibitors may increase exposure to the MMAE component and raise the risk of toxicities. Patients with moderate renal impairment appear not to have substantially altered pharmacokinetics, while data in severe renal impairment are limited. Patients should be educated about signs of neuropathy and infection. Careful consideration is needed when combining with other immunosuppressive therapies.
Expert Tips
Prescribers should ensure eligibility criteria are met, including prior therapy status and prognostic risk scores when choosing Polatuzumab Vedotin. Close monitoring of hematologic function and prompt management of infusion reactions are critical. Pharmacists should counsel patients on recognizing symptoms of neuropathy, ensuring supportive care for GI side effects, and maintaining schedule adherence. Pre-treatment evaluation including hepatic assessment and ensuring infection prophylaxis where standard is helpful to reduce complication risk.
