Semaglutide
Overview
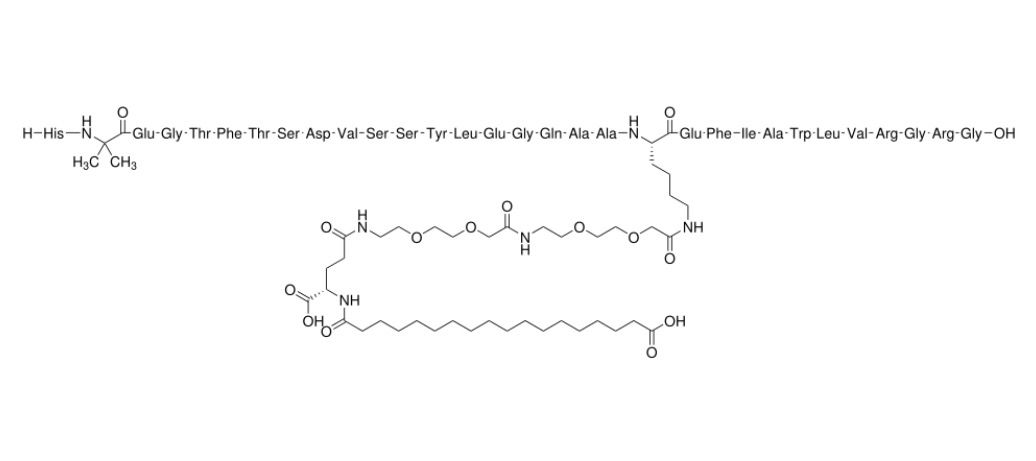
Semaglutide is a long‑acting glucagon‑like peptide‑1 (GLP‑1) receptor agonist, designed to mimic the natural hormone GLP-1, which regulates blood sugar and appetite. Thanks to a fatty acid-based modification, it binds strongly to albumin and resists degradation, giving it a prolonged effect in the body. This enables once-weekly injectable or once-daily oral formulations. As a precision metabolic therapy, semaglutide helps improve glycemic control, supports weight management, and reduces cardiovascular and kidney-related risks in certain patients.
Background and Date of Approval
Semaglutide, developed by Novo Nordisk, shares high sequence homology with native human GLP-1, but its design gives it a long half-life, making weekly or daily dosing possible. The subcutaneous form was first approved for type 2 diabetes and later for weight management and cardiovascular risk reduction. The oral tablet formulation was also developed to allow patients who prefer not to inject to take semaglutide by mouth. Clinical trials demonstrated significant improvements in glycemic control, weight reduction, and cardiovascular outcomes in targeted patient populations.
Uses
Semaglutide is indicated for adults with type 2 diabetes to improve blood glucose control, particularly when diet and exercise alone are insufficient. It is also indicated to reduce the risk of major cardiovascular events in adults with type 2 diabetes and established cardiovascular disease. For weight management, high-dose semaglutide is used in patients with obesity or overweight with weight-related conditions, helping reduce appetite and promote weight loss. Treatment requires individual assessment and monitoring for efficacy and safety.
Administration
Semaglutide can be given as a subcutaneous injection or taken orally, depending on the formulation. The weekly injectable dose is gradually escalated in many weight-management regimens, while a stable maintenance dose is used for diabetes therapy. The oral tablet is available in multiple strengths and should be taken according to the prescribed schedule. Patients may take semaglutide with or without food. Treatment usually continues until therapeutic goals are met or until the clinician determines that efficacy or tolerability is not acceptable.
Side Effects
Common side effects of semaglutide include nausea, diarrhea, vomiting, abdominal pain, constipation, and reduced appetite. Many gastrointestinal side effects tend to occur during treatment initiation or dose escalation and may lessen over time.
Warnings
Semaglutide has serious risk considerations, including pancreatitis, diabetic retinopathy complications, potential thyroid C-cell tumors in high-risk patients, and severe hypoglycemia when used with insulin or other glucose-lowering therapies. The oral form may also cause kidney problems, and patients should be monitored for changes in urine output or fluid retention.
Precautions
Semaglutide should not be used in individuals with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2. Because it slows gastric emptying, it may alter the absorption of oral medications. Semaglutide may interact with other glucose-lowering drugs, requiring dose adjustments and careful monitoring. Patients should be monitored for kidney, thyroid, and pancreatic health throughout therapy.
Expert Tips
When prescribing semaglutide, counsel patients about gradual onset of side effects, particularly nausea, and advise dose escalation as per protocols. Monitor renal function, thyroid status, and eye health. For injectable semaglutide, rotate injection sites to prevent local reactions. With oral semaglutide, ensure patients follow instructions carefully to optimize absorption. Review all co-administered medications to prevent interactions and ensure therapy continues safely until treatment goals are achieved.
